Watershed Detectives - Background
Background: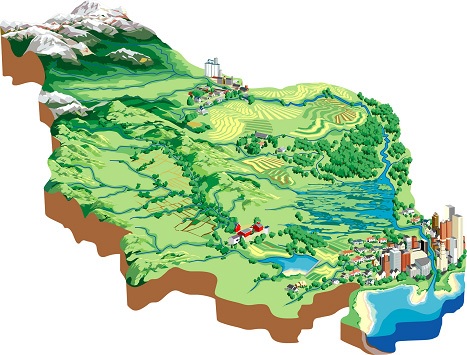
A watershed is an area of land from which all the water drains to the same location, such as the land that all drains to a stream, pond, lake, river, wetland or estuary. A watershed can be quite large, such as the Colorado River drainage basin, or very small, such as a small horse pasture that drains to a farm pond. Watersheds are “nested”, with many small watersheds containing larger watersheds. Watersheds are comprised of upland areas, riparian areas (the strips of water loving vegetation near streams, lakes and other water bodies), and the streams, lakes and wetlands that collect and transport the water and anything carried by the water.
The natural conditions of a watershed, such as its elevation, annual precipitation and temperature, native geology and plant communities will all determine the quality of the water leaving that watershed. “Point sources” of pollution are discharges from industries or waste water treatment plants and may be significant in some areas. In many watersheds, however, streams and lakes are more affected by “nonpoint source pollution” which enters primarily from rain and snowmelt runoff over the land surfaces. The amount and types of pollutants are determined by the land uses and activities in a watershed. Different types of land uses and activities, such as roads and urban development, mining, timber harvesting, recreation, and agricultural activities, may result in quite different mixes and amounts of pollutants. Nonpoint source pollution is associated with rainfall and snowmelt runoff moving over and through the ground, carrying natural and human made pollutants into water sources. Examples of nonpoint source pollutants are fertilizers, pesticides, sediment, gas, and oil. Pollutants such as nutrients, pesticides, oil and gas products, salts, sediment and bacteria can drastically alter the state of the stream or lake's ecosystem. If we can determine the type of pollutant and its source, we can take preventative measures to reduce any further contamination.
Students will make the following measurements on each water sample:
| Turbidity: Turbidity is a measure of suspended material (such as sediment or microorganisms) in the water. Suspended sediment eventually settles out in a stream and fills in the spaces between the rocks and gravel on a stream bottom. This can suffocate the tiny aquatic animals living between the rocks or the fish eggs laid on stream bottoms. Turbidity can also prevent sunlight from reaching aquatic plants and may also affect the ability of fish and aquatic invertebrates to see and capture their prey. Turbidity increases naturally when flows increase, but also increases from uncontrolled runoff from agricultural fields, roads and trails or construction sites. Also, nutrients can increase turbidity. How to measure turbidity. | |
| Nitrate (NO3): This is the most common form of inorganic nitrogen in unpolluted waters. It is an essential nutrient for plant and animal growth. However, increased amounts of nitrate can lead to excessive plant growth which can decrease the aesthetic value of water bodies (by making it murky, smelly or creating a slimy bottom). The decomposition of this extra plant material also uses up oxygen in streams and lakes, resulting in fish kills. Sources of nitrate include fertilizers, animal waste and failing septic systems. How to measure nitrate. | |
| pH: This is a measurement of how acidic or basic something is. pH is measured on a scale from 0 to 14 with 7 being neutral. The lower numbers on the scale are more acidic, while the higher numbers are more basic. The pH scale is logarithmic, which means each unit change (e.g., from 7 to 8) in pH represents a 10-fold change in the acidity. How to measure pH. |
Students will also be given information on other measurements that are more difficult
to modify in a classroom setting. These include:
Dissolved Oxygen: This is not the bubbles in water, or the oxygen part of the H20 water molecule. It is a separate oxygen molecule that is dissolved into water. It gets into the water either by oxygen from the atmosphere mixing into a river where there is turbulence, or by aquatic plants releasing oxygen during photosynthesis. Fish and aquatic macroinvertebrates require a certain level of dissolved oxygen in order to survive.
Temperature: The temperature of water is the amount of heat energy it contains. Temperature can be measured in Fahrenheit or Celsius. Since state requirements are usually in Celsius, that is the preferred scale for testing water samples.
Preparing Water Samples:
These measurements are for 1 gallon water samples. Measurements are estimates and may vary due to differences in tap water.
Gold Creek
High Turbidity: Add a pinch of silt/soil or a small dash of modeling clay until the disc at the bottom of the turbidity tube can be seen between 25 and 30cm.
Low Nitrates: No addition needed.
Low pH: Add vinegar or lemon juice until pH reaches 4 - 5 (approximately 1T of vinegar).
Straight Shot Stream
High Turbidity: Add a pinch of silt/soil or a small dash of modeling clay until the disc at the bottom of the turbidity tube can be seen between 25 and 30cm.
High Nitrates: Add sodium nitrate until the concentration is between 1.5 and 2mg/liter (approximately 20 grains).
Neutral pH: Add baking soda until pH is 7 (approximately 1/4T).
Red Ribbon River
High Turbidity: Add a pinch of silt/soil or a small dash of modeling clay until the disc at the bottom of the turbidity tube can be seen between 25 and 30cm.
High Nitrates: Add sodium nitrate until the concentration is about 5mg/liter (approximately 40 grains).
Neutral pH: Add baking soda until pH is 7 (approximately 1/4T).
Capital Creek
Medium Turbidity: Add slightly less than a pinch of silt/soil or a very small dash of modeling clay until the disc at the bottom of the turbidity tube can be seen between 35 and 40cm.
Low Nitrates: No addition needed.
Neutral pH: Add baking soda until pH is 7 (approximately 1/4T).
Off Road Dream Stream
High Turbidity: Add a pinch of silt/soil or a small dash of modeling clay until the disc at the bottom of the turbidity tube can be seen between 25 and 30cm.
Low Nitrates: No addition needed.
Neutral pH: No addition needed.
Mayfly River
Low Turbidity: No addition needed.
Low Nitrates: No addition needed.
Low-Neutral pH: Add vinegar or lemon juice until pH is between 6 and 6.5 (approximately 1/2T vinegar).
Back to Watershed Detectives main page.
