Nitrogen Cycle - Apply the Data
There are multiple factors that affect the toxic concentrations of ammonia to fish, including the pH, temperature, and exposure times.
Ammonia
The toxicity of ammonia to fish and other aquatic life is affected by the pH of the
water, by the temperature of the water, and by how long the fish are exposed to ammonia
in the water.
pH Effects
The common form of the ammonia molecule has one nitrogen and three hydrogen atoms
(NH3). This form is toxic to fish at very low concentrations. In somewhat acidic water,
however, a fourth hydrogen atom attaches to ammonia, creating the ammonium ion (NH4+).
Ammonium is much less toxic to fish. At a neutral pH the ammonia concentration is
higher than it would be at the same temperature with a lower pH. Therefore, we need
to know the pH of water to know how toxic the ammonia concentration really is.
Temperature Effects
At temperatures above room temperature (about 20 degrees C), for both forms of ammonia
become more toxic to fish.
Exposure times
Most fish can handle higher concentrations of ammonia for short periods of time (hours
or less) than over longer periods of days and weeks.
Here is a graph showing the effect of temperature and pH on ammonia toxicity to fish.
This is over a short term exposure (just a few hours).
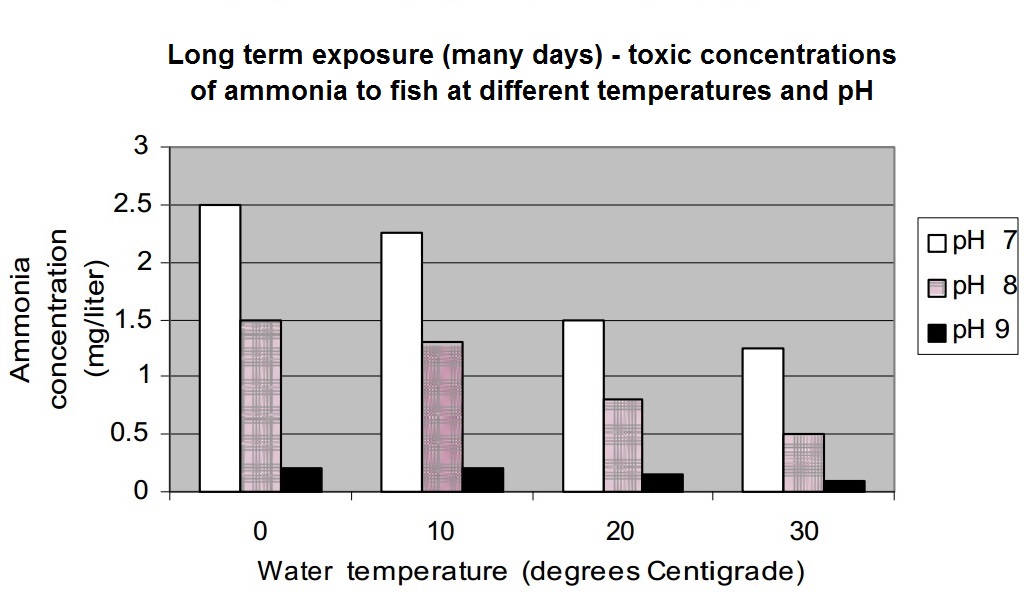
Discussion:
- Notice how ammonia becomes much more toxic a the water's pH increases from 7 to 9. For example, at water temperatures of 10 degrees C, the toxic concentration at pH 7 is 21 mg/liter, but at pH 9, the toxic concentration is 1 mg/liter.
- A change from pH 7 to 9 could occur within a single day in some streams.
Here is a graph showing the effect of temperature and pH on ammonia toxicity to fish. This is over a long term exposure (many days).
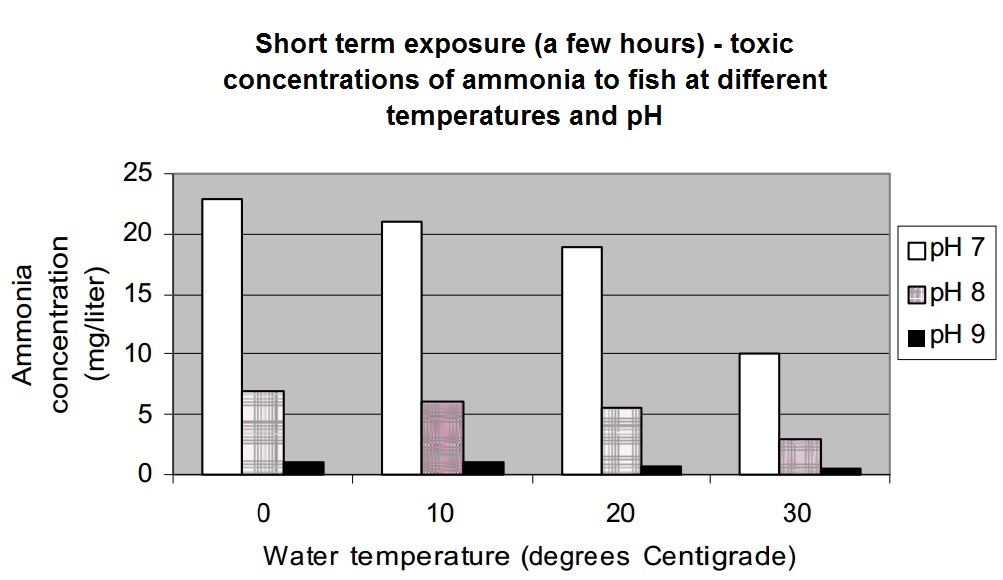
Discussion:
- Compare this graph to the one above. Notice that at the same pH and temperature, much lower concentrations affect fish exposed for long periods of time.
- Notice how the concentration at which the ammonia becomes toxic is lower as the temperature increases. For example, at a pH of 7, the toxic concentration is 2.5 mg/liter at 0 degrees C, but at 30 degrees C the toxic concentration is 1.2 mg/liter. Luckily, temperatures this high are not often found in natural streams.
From Utah Stream Team (pages 105 to 106)
